We take a deep dive to understand how chemistry works at a fundamental level. All chemistry is about electrons. They are the primary drivers responsible for an atom’s behavior in chemical reactions, or more precisely the way electrons can be configured in atoms, which is determined by the Schrodinger equation, developed by physicist Erwin Schrödinger in 1925.
The Schrödinger equation introduced the concept of thinking of electrons as waves. The solutions to the Schrödinger equation provides us with atomic orbitals, or regions in the space around the nucleus where the probability of finding an electron is highest. These orbitals form the basis of electron shells and subshells (s, p, d, f), each with a unique shape and energy level. The outermost subshells, or valence shells, are the underlying reason that the periodic table is structured the way it is.
The elements on the far right of the table are the noble gases, which have their outer p shells full, which makes them stable. Since their outermost shell is already full, they are less reactive. Every other element is not as stable. But by either sharing or transferring electrons between or among atoms, the entire system can become more energetically stable.
Electronegativity is a measure of the tendency of an atom in a molecule to attract electrons in the system towards itself. When atoms with different electronegativities bond, the electrons in the molecule tend to be more attracted to the more electronegative atom. This creates a slightly positive and negative polarity within the molecule, but if the electronegativity difference is extreme, the more electronegative element can effectively strip the electron(s) completely from another atom. This is what happens with acids. Flourine is the most electronegative element and can form very strong acids. For example hydrogen fluoride, the fluoride atom strips the electron completely away from the hydrogen forming a lone proton, or H+ in aqueous solution.
An acid is a substance that donates a proton (H⁺), and a base is a substance that accepts it. So HCl dissolved in water is an acid because it donates a proton to a water molecule, forming hydronium ions which are water molecules with an extra proton, creating a positive charge. HCl is the acid and water is the base.
When an acid donates a proton, it creates a conjugate base because after donating the proton, it can gain a proton. While the base that gains the proton becomes a conjugate acid, because after gaining a proton, it can donate it. For HCl dissolved in water, HCl is the acid, water is the base, Cl⁻ is the conjugate base, and H₃O⁺ is the conjugate acid.
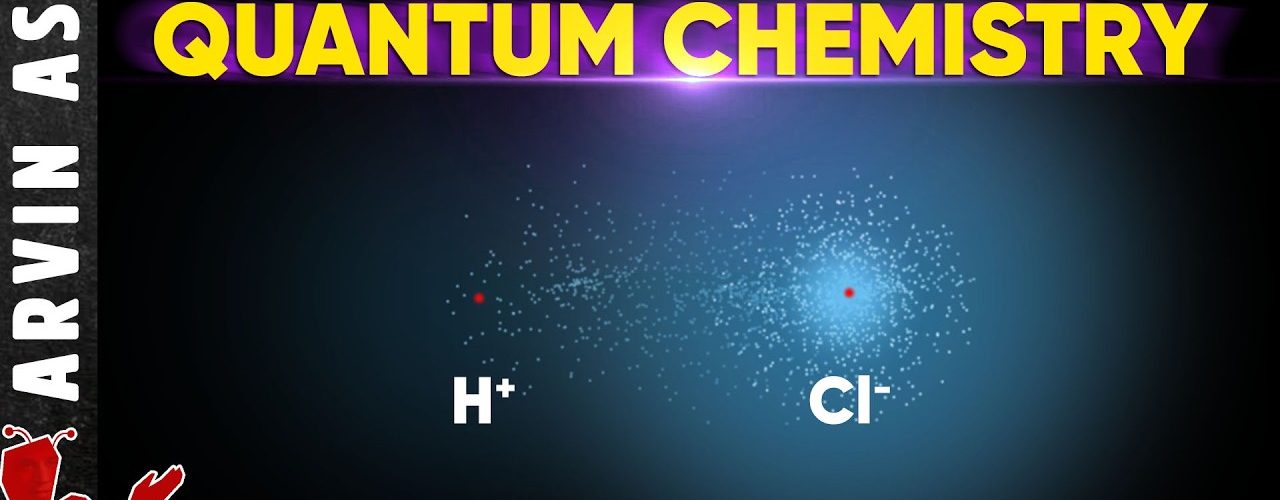
From a quantum mechanics perspective, the tendency of acids like hydrochloric acid (HCl) to “give away” or donate their proton (H⁺) is related to the electron density distribution in the molecule and the nature of the chemical bond between hydrogen and chlorine atoms. The wavefunction describing the electron cloud around H and Cl in HCl shows that the electron density is predominantly located around the chlorine nucleus, leaving the hydrogen atom with a very small electron density, nearly resembling a free proton.
Because of this, the hydrogen is easily dissociated as a positively charged proton in an aqueous environment or in the presence of a base that can accept it. This process is also stabilized by the chlorine atom, which has a lone pair of electrons with high electron density, allowing it to exist as a stable chloride ion (Cl⁻) after H⁺ or proton is donated.
#acidbasechemistry
#quantummechanics
#quantumchemistry
The likelihood of proton donation in acids like HCl is rooted in the quantum-mechanical behavior of electrons in the molecule, where the electron cloud’s asymmetry and the stabilizing effect of the electronegative oxygen atom in water allows the proton to dissociate readily. That’s what makes HCl a strong acid. The strength of an acid is measured by how strongly it can donate protons.