In Ancient Egypt, the sun was worshipped as a god – Ra, the ruler of the skies, and earth, and bringer of light and life. Similarly, in ancient North America, almost every tribe has some symbol for the sun and puts it in high regard as the bringer of light, warmth and food.
While it is true that the sun does all those things, and without it we could not survive, it is not a being or mysterious object that we don’t understand.
As much as we would like to believe the anthropic view that the sun is here for our benefit, it is really just trying to survive. It is in a constant battle, the battle between the crushing force of gravity that would have it collapse in on itself, and the energy it needs to create in order to counteract this inward pressure.
We have a very good idea of what the sun is made of and how it works. Let’s talk about the second most important celestial object in the universe. You are living on the first.
What is fundamentally going on inside? Why isn’t the sun like a log in a fireplace, that burns out after a short period of time. Why and how does the sun keep on creating so much energy. And how long will its fuel last?
To a scientist living in the 18th century, the most likely analogy of how the sun worked might have been to compare it to a log in a fireplace. When something like wood, burns there is a reaction between it and oxygen in the air that creates energy in the form of heat and light.
Could some kind of burning be the mechanism of the sun’s light? When a log burns, it releases about 1 ten-millionth of a trillionth of a watt per atom.
The mass of the sun was fairly well understood because it can be calculated using the known orbits of the earth and other planets, and Newton’s laws of universal gravitation. And based on this, we can calculate the sun has a mass of about 2 x 10^30 kg. And this translates to about 10^57 atoms.
And the sun puts out a lot of energy. If everyone on earth held turned on 1 million, 100 watt light bulbs, it would still be 10 billion times less than the luminosity of the sun.
The sun in fact produces about 384 septillion watts.
And if you take the number of atoms and figure out how long it would continue to produce this amount of energy similar to the efficiency of wood, you can calculate this number to be about 20,000 years.
This, of course cannot be the mechanism of the sun shining because at this rate, it would have burned out a long time ago.
We know from radioactive dating of meteorites which are part of the leftover remnants from the sun’s original formation, that the sun is at least 4.5 billion years old.
So what does cause the sun to shine at such high energies for so long? Where does this energy come from? Well it comes from a little equation you may be familiar with: E=MC^2.
This equation which comes from Einstein’s theory of special relativity, although quite simple, tells us something quite profound. In words, it really says that energy is equal to mass times a conversion factor, which is C, the speed of light squared. This is just a big number.
So what it is telling us that energy and mass are equivalent. They are essentially the same thing. In fact, a little bit of mass is equivalent to a lot of energy. In other words, lots of energy can be gained from using up a modest amount of mass, because squaring C is which is equal to, 300,000,000 m/sec is equal to a very large number: 9 X 10^16 m^2/sec^2
So based on the mass of the sun, how much energy does it contain. Well, we have to be careful with this calculation because the sun doesn’t have access to all the energy contained its mass. If it did, in other words, if it could convert all its mass 2 x 10^30 kg to mass, this would amount to 1.8 x 10^47 joules. At a consumption rate of 3.8 x 10^26 joules per second, the sun would burn for 15 trillion years.
But the sun doesn’t convert all its energy to mass. It converts only about 0.7 percent of its mass to energy in a process called fusion. This is when 4 hydrogen atoms fuse to form one helium atom. And using this process, the sun will last approximately, 10 billion years. It is already middle aged since it is about 4.5 billion years old.
Keep in mind that fusion is not like the chemical process of bonding atoms together to form molecules, such as 2 hydrogen atoms and one oxygen making a water molecule, but it is a conversion of one element to another element by changing the number of protons and neutrons in the nucleus.
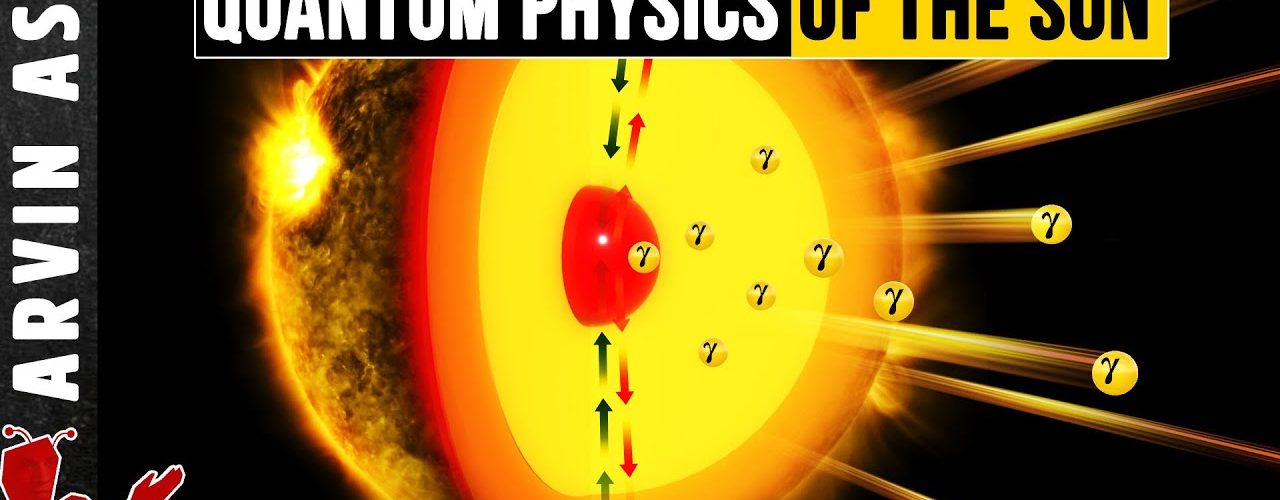
This is not a process that we normally observe on earth because it requires enormous pressures and temperatures – the kind of environment available in the centers of stars. This fusion of hydrogen nuclei to make a helium nucleus converts some of the mass to energy. And this is the energy that you see from the sun in the form of photons.
So how does fusion work?
You would think that since the protons that make up the nuclei of the 4 hydrogen atoms are positively charged, they would repel each other. You would be right. This due to the coulomb force. This is what keeps atoms from spontaneously fusing.
The protons have to overcome an energy barrier. Once they get over this barrier, and get very close to each other, the attractive force of the strong nuclear force takes over and glues them together.
The strong nuclear force is about 100 times stronger than the electromagnetic force pushing protons apart, but it acts at only a very short range – about the width of the proton. So how do the protons get close enough for this to happen?
Inside stars like the sun, there are a couple of things which allow this fusion to happen. First the extreme temperature, about 15 million degrees Celsius, rips atoms into their components: protons, neutrons and electrons.
Extreme pressures also force these components to be in close proximity to each other. This is good enough for fusion to initiate, but to keep things going, the sun has another trick up its sleeve.
This trick is called quantum tunneling. This is due to the uncertainty principle in quantum mechanics.
One way to write this equation is that the uncertainty in energy times the uncertainty in time is equal to a constant. Basically, if particles borrow energy and give it back for a short enough time, it’s as if the universe doesn’t record it. It can’t be measured. But this is part of quantum mechanics.
So the particles can borrow energy for very short periods of time, such that it tunnels through the coulomb barrier, as if it is not even there. So instead of going over the hump, it goes straight to the other side. This happens in quantum mechanics, usually when there is a lower energy state on the other side of the barrier. This quantum tunneling effect dramatically improves the sustainability of fusion in the sun.
In the hydrogen to helium fusion process, first two protons fuse. Usually, the pair breaks apart again immediately, but once in a while one of the protons is transmuted into a neutron. This happens due to the weak nuclear force in a process called beta decay. A neutron turns into a proton by releasing an anti-electron or positron and a neutrino. The resulting proton-neutron pair is deuterium, an isotope of hydrogen.
When the positron emitted from the beta decay encounters its antiparticle (an electron), the pair annihilates to form a gamma ray, which is the most energetic form of photons. Gamma rays eventually work their way up from the core of the sun and out into space in the form of sunlight. The deuterium nuclei can merge to form helium nuclei (He-4), or they can interact with other protons to make another isotope of helium (He-3). Two He-3 nuclei can fuse to make an unstable beryllium nucleus (Be-6) that breaks apart to give He-4 and two protons. Energy is released at each step.
So overall, we start with 4 hydrogen nuclei, or 4 protons, and end up one Helium nucleus, or 2 protons and 2 neutrons. This is really a fusion of our 4 original protons. Two of them have just transmuted to neutrons in a beta decay.
You might be asking at this point, where does the energy come from? This is where Einstein’s equation E=MC^2 comes into the picture. Mass is converted to energy.
If we look at the atomic weight of 4 hydrogen atoms we see that it is equal to 4 x 1.008 or 4.032. Just a little over 4. A helium atom has atomic weight of 4.0026. You’ll notice that there is a small difference. The 4 hydrogen atoms weigh slightly more than one Helium atom. The difference is about 0.0294 or about 0.7% less.
And this tiny difference is responsible for nearly all the energy that you see coming from the sun. And it is the source of the pressure that keeps the sun from collapsing in on itself, just like a pot of water puts pressure against the lid.
This is the extent of the explanation you will hear in most tutorials. But we are quantum physicists, so let’s go one step deeper. You get that the energy comes from the reduction in mass. But where does this reduction in mass come from?
A proton has a mass in units of energy, of 938.3 MeV. A neutron has a mass of 939.6 MeV. Since a Helium atom is made of 2 each. If you add them up, the total mass comes out to be 3755.8MeV. That’s what it should be if all its constituents added together, but that isn’t the mass.
The measured mass of the Helium atom is actually 3728.41 MeV. That’s a difference of 27.48 MeV, or 0.7%. So what happened? Well, it turns out that the 4 nucleons which comprise the nucleus of the Helium atom are more stable together than when they existed apart on their own. This higher stability is where the energy releases energy.
This is a result of the fact that Helium has higher binding energy than Hydrogen. The terminology binding energy is a bit confusing, because it seems the opposite of what it should be.
But basically what binding energy means is the amount of energy needed to pull the nucleons apart. In other words, it’s the energy needed to pull the two protons and two neutrons apart, or free them up, from the nucleus. You would have to put energy in to pull nucleons of helium apart.
Hydrogen has zero binding energy because there is only one proton in the nucleus. There is nothing to pull it apart from.
The difference in binding energy between Helium and hydrogen is the source of the energy released in the fusion process.
Let’s go one step deeper, because we are very curious quantum physicists. Why are the 4 nucleons of helium more stable together than when they are separated apart? Now we have gotten to the crux of the source of energy.
You have to understand where mass comes from in the first place. Most of the mass does not come from the Higgs field. Only about 1% of the mass is due to the Higgs field. How you ask?
The protons and neutrons are made of 3 quarks. Two up quarks and one down quark for the Proton. Two down and one up quark for the Neutron. The Higgs field gives mass to these quarks. If you add up the masses of these three constituent quarks, it’s the following, whereas the mass of the nucleon is about 938 MeV. So the quarks add up to only about 1% of the mass of the nucleon.
So where does the rest of the energy come from? Well, it comes from one of the fundamental forces of the universe – the strong force.
This force is the glue that keeps quarks bound to the nucleus. It is mediated by a fundamental particle called the gluon.
The combination of quarks and gluons inside the nucleon forms a kind of plasma – called the quark gluon plasma consisting of quark-gluon-quark interactions.
They are moving at very high speed, and a lot of energy is bound up not only in the energy of their movement, but also energy that binds the plasma together.
I made an intuitive video about this using Feynman diagrams if you want to see the details, take a look at that video.
But a simplified picture looks like this. It so happens that when multiple nucleons are together, there is less energy required to keep these quarks and gluons bound to within the nucleon. This is because the quarks and gluons are now being constantly exchanged with neighboring nucleons. They can move in and out of the nucleon more easily.
To be more precise, quarks are confined to the nucleon, but as they gain more energy, more quarks are created. The exchange of quarks between nucleons is made by Mesons, which are quark anti-quark pairs, which get formed in the process. These Mesons mediate the strong nuclear force which keeps these nucleons glued to each other.
This requires less energy overall than when these quarks and gluons have nowhere to go, for example in the hydrogen atom, where the Proton is by itself with no other nucleons to exchange its quarks with.
So this is why a helium atom with 4 nucleons is more stable than the nucleons on their own. This stability is mostly due to binding energy mediated by the strong force.
And that is the quantum mechanical explanation of why the sun releases so much energy which we see in the form of sunshine.
And the energy coming from this helps the sun fight the battle for its survival. This battle that it undertakes is really on our behalf because the competition between gravity and outward pressure creates the energy that powers life on earth. And without it we would not exist.
And although the sun will continue to win the battle for at least 5 billion more years, it will eventually lose the war because the fuel will run out, and the sun will collapse into a white dwarf about the size of the earth, and with the luminosity of not much more than the moon, and then will eventually become dark and die.
But until then, let us enjoy a few more cosmic minutes of basking in the sun. I’d like to thank my generous supporters on Patreon. If you like videos like this, consider joining them. And leave a question in the comments if you don’t understand something. I will do my best to answer it. I will see you in the next video my friend!